DCGI approves two coronavirus vaccines

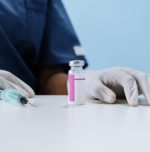
The Drugs Controller General of India (DCGI) yesterday approved two vaccines against COVID-19, one is made by Serum Institute and another by Bharat Biotech. The DCGI in this context said that Oxford COVID-19 vaccine, Covishield got approved for initial vaccination while the indigenously developed Covaxin has been approved for restricted use.
As there has been an increase in coronavirus cases, and especially the new mutant strain infection has been increasing in the country, Covaxin got approval from the DCGI for emergency use.
Bharat Biotech’s Covaxin did not finish the third phase of clinical trials. In this context, opposition parties questioned its safety. Hence, AIIMS Director Dr Randeep Guleria clarified that this vaccine would be used only when an emergency need arises due to the increasing coronavirus cases.
He also said that Covaxin could be used as a backup since the efficiency of Covishield was also not fully known. Even though Covaxin got approval for emergency use, the company will continue the third phase trials to understand its safety and efficiency.
Serum Institute’s Covishield would be given in the first phase of vaccination. Meanwhile, Covaxin will finish the remaining clinical trials after which it will be used for mass drive.
Both vaccines need cold storage as they need to be stored at 2-8 degrees Celsius.
Guleria said the vaccine would be rolled out in the next two weeks. He added that cold storage has to be used properly to avoid wastage of vaccines.
In addition to Covishield and Covaxin, the DCGI also approved Cadila healthcare for phase 3 clinical trials. Besides, the Serum Institute, in association with Novavax, has been developing another vaccine. Thus, India keeps four vaccines ready. While two are ready and another two are getting ready to be used soon.
Image by Markus Winkler from Pixabay (Free for commercial use)
Image Reference: https://pixabay.com/photos/typewriter-paper-covid-19-vaccine-5521552/







Leave a Reply