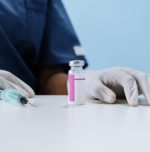
Corbevax approved for EUA in 12-18 year children

Biological E’s Corbevax has been granted emergency use authorisation (EUA) from the Drugs Controller General of India (DCGI) to be used in children aged 12-18 years.
Corbevax is the third vaccine approved for children after Bharat Biotech’s Covaxin and Cadila Healthcare’s ZyCoV-D.
Covaxin has already been used for vaccinating children aged 15-17 years. Last October, the Subject Expert Committee (SEC) recommended approval of Covaxin in kids aged two years. But, the DCGI approved it to be used in children aged 12-18 years.
Regarding ZyCoV-D, it is not yet used for adolescents even though it got approval for their use. The vaccine has been used only in adults as of now.
As per sources, the National Technical Advisory Group on Immunisation in India (NTAGI) wanted to see the results in adults before administering the vaccine to children. As the vaccine did not show any big complications in adults, the vaccine will be given to children soon.
Now, Biological E’s Corbevax got emergency use authorisation for children, based on the clinical trials of phase-2 and 3. However, the administration of the vaccine to children lies with the decision of NTAGI.
As Corbevax has already been approved for adults, Biological E started supplying the vaccine to the government as it placed an order for 300 million vaccine doses.
Corbevax is a protein subunit vaccine. This two-dose vaccine is administered through an intramuscular route. Dr NK Arora, COVID-19 Task Force Chairman of NTAGI, says that its antibody level is very high.
Currently, children above 15 years are getting vaccinated in India. The vaccination for children below 15 years has not yet started.
Nearly 70 per cent of children in the age group of 15-17 years received at least one dose. More than 2 crore children have been fully vaccinated. There are over 7 crore children in the age group of 15-18 years for 2021-22.
Image by Shafin_Protic from Pixabay (Free for commercial use)
Image Reference: https://pixabay.com/vectors/covid-19-vaccine-corona-virus-5358852/






Leave a Reply