Licenses of 18 Firms cancelled for making spurious drugs
The Indian pharmaceutical industry has faced scrutiny in recent years due to concerns over the quality of drugs manufactured by companies.
In a major crackdown against the production of substandard drugs, central and state regulators in India conducted joint inspections at 76 pharma companies across 20 states and Union territories in the past 15 days.
The inspections were conducted as part of a special drive against the manufacture of spurious and adulterated drugs, resulting in the cancellation of licenses for 18 pharma companies and the issuance of show-cause notices to 26 other firms. The majority of the identified companies are from Himachal Pradesh, followed by Uttarakhand, and Madhya Pradesh.
This crackdown follows various incidents that have raised questions about the quality of drugs produced by Indian pharmaceutical companies.
For instance, earlier this year, Tamil Nadu-based Global Pharma Healthcare recalled its entire lot of eye drops that were allegedly linked to vision loss in the United States. Additionally, last year, India-made cough syrups were allegedly linked to children’s deaths in the Gambia and Uzbekistan.
Moreover, in February 2022, nine medicines manufactured by Himachal-based pharma companies failed to meet safety standards tests of the Central Drugs Standard Control Organisation, India’s apex drug regulator.
The drugs that failed the test include favipiravir used for the treatment of COVID-19 and other drugs used in the treatment of heart attack, gastric issues, gout, and high blood pressure.
As a result, the Himachal Pradesh drug controller issued notices to the nine companies and asked them to withdraw entire batches of these medicines from the market.
Earlier in July 2021, Himachal’s pharmaceutical industry was accused of illegal drug rackets, and the Punjab Police raided units at Paonta Sahib and Kala Amb in the Sirmaur district.
They found that tramadol, an opioid painkiller, manufactured by Orison Pharma, was being marketed by Mumbai-based PP Pharma that only existed on paper.
The Punjab Police made arrests and recovered 30 lakh capsules valued at ₹15 crores. Such incidents continue to worry the leading manufacturing companies in Himachal along with the state government.
The recent crackdown by regulators on the manufacture of substandard drugs is an important step towards improving the quality of pharmaceutical products in India.
It is crucial to ensure that patients receive safe and effective medications and that the reputation of the Indian pharmaceutical industry is maintained.
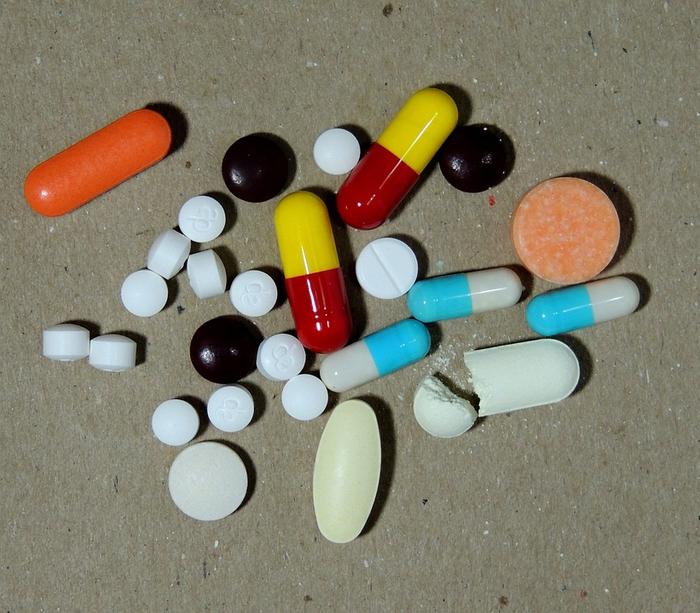
Image by Junior Peres Junior from Pixabay (Free for commercial use)
Image Reference: https://pixabay.com/photos/medicines-pills-diseases-423832/







Leave a Reply